Acetic Acid React With Sodium Carbonate
Sodium acetate is formed after the reaction between a strong base sodium hydroxide a strong base and acetic acid a weak acid. Rearrange the expression to solve for the hydronium ion concentration.

Question 10 Mcqs From Ncert Exemplar
The white precipitate of strontium carbonate formed in the group analysis dissolves in hot dil.

. Which of the following is an acid salt. Which of the following is a diacidic base. A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and its salt.
Salicylic acid can react with acetic ethanoic acid in an esterification reaction but the reaction is very slow taking days to reach equilibrium. Some primary standard acids are potassium hydrogen phthalate KHP oxalic acid dihydrate sulfamic acid and benzoic acid. Which of the following acid is a dibasic acid.
Acetic acid reacts with ammonium sulphate to form a white precipitate o strontium sulphate. Strontium acetate formed by dissolving Strontium carbonate in dil. Indeed the versatility of water as a solvent is essential to living organisms.
Acetic acid undergoes nearly all carboxylic acid reactions. Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. An example of a buffer that consists of a weak base and its salt is a solution of ammonia and ammonium chloride NH 3 aq NH 4 Claq.
A The buffered solution on the left and the unbuffered solution on the right have the. 6Li s N2 g 2Li3N s How many moles of lithium are needed to produce 045 mol of Li3N when the reaction is carried out in the presence of excess nitrogen. First write the equation for the ionization of acetic acid and the K a expression.
Acetic acid due to the formation of soluble strontium acetate. Vinegar is no less than 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. When a strong acid reacts with a weak base the base is.
It is one of the most plentiful and essential of compounds. Assuming the change in volume when the sodium acetate is not significant estimate the pH of the acetic acidsodium acetate buffer solution. Acetic acid undergoes decomposition when heated above 440C to yield either methane and carbon dioxide or water and ethanone given by the equations.
Acetylsalicylic acid sodium hydrogen carbonate a salt sodium acetylsalicylate carbon dioxide gas water NaHCO 3aq CO 2g H 2 O l C 9 H 8 O 4s NaHCO 3aq C 9 H 7 O 4s-Na CO 2g H 2 O l The. Cause of formation of acidic basic and neutral salts. A Ammonium sulphate test.
Two common primary standard bases are pure sodium carbonate and borax. Acetic acid is a polar protic solvent with a dielectric constant of 62 in its liquid form. The K a for acetic acid is 17 x 10-5.
The primary standard acid in this experiment is KHP 1 which is the monoprotic potassium salt of a diprotic carboxylic acid KHC 8H4O4. A tasteless and odourless liquid at room temperature it has the important ability to dissolve many other substances. Lithium and nitrogen react in a combination reaction to produce lithium nitride.
The molecular weight of the acetic acid CH3CO2H rounded to the nearest integer is _____ amu. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states.

Sodium Carbonate Reacts With Ethanoic Acid To Form Sodium Ethanote Caron Dioxide And Water In An Youtube

Cbse Class 10 Science Lab Manual Properties Of Acetic Acid A Plus Topper
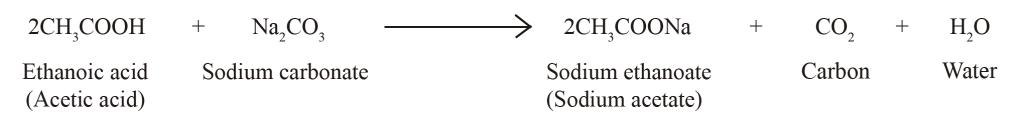
No comments for "Acetic Acid React With Sodium Carbonate"
Post a Comment