Which of the Following Conformers Has the Highest Energy
We review their content and use your feedback to keep the quality high. Which is the highest energy conformer of 23-dimethylbutane.

Which Of The Following Is The Highest Energy Conformer Of The Following Compound Looking Down The Indicated Bond Explain Study Com
The substituents present in axial positions cause the steric strain as a result the molecule is more unstable.

. H3C Lowest Energy H CH3 2nd Lowest Energy 4. The most stable conformer for 3-methylpentane viewed along the C_2 - C_3 bond will have the ethyl group anti to a methyl group with a methyl - methyl gauche interaction. C A staggered conformation with two larger groups 60 from each other is called anti.
Recall the more stable the conformer the lower the energy. Match each conformer to their corresponding relative energy. The answer is 3.
Which of the following statements about constitutional isomers is NOT true. D Gauche conformations are generally higher in energy than anti conformations. B Staggered conformations are at energy maxima and eclipsed conformations are energy minima.
IV Rank the conformers of 1 2 4-trimethylcyclohexane in order of. Im gonna label the Thedc carbon that has the chlorine substitution as number one and then the carbon with the muscles of a situation as number 23 or four whatever its going. There is one low-energy conformer of trans-decalin vs.
This problem has been solved. Chemistry questions and answers. So start with the bond line notation for 3-methylpentane Notice that you have two hydrogens and a methyl group attached to C_2 and one hydrogen one methyl and one ethyl group attached to C_3.
Who are the experts. Compare the following conformers of butan-2-ol. A The highest energy conformer is one in which methyl groups are eclipsed by hydrogens.
C A staggered conformation with two larger groups 60 from each other is called anti. H CH3 ČH3 check_circle Expert Answer. This problem is asking us which of these conference has the lowest stability.
This is due to a crowding of the two methyl groups in the gauche structure and is. 21 In the lowest energy chair conformation of cis-13-dimethylcyclohexane how many axial positions are occupied by hydrogen atoms. Previous question Next question.
The highest energy conformer the eclipsed conformer maximizes the repulsions between the C-H bonds on one carbon and the C-H bonds of the adjacent carbon resulting in an energy of 35803 kcal mol. Staggered conformations are at energy maxima and eclipsed conformations are energy minima. The highest energy conformer is one in which methyl groups are eclipsed by hydrogens.
Because B is lower in energy than A ie more stable B is the major isomer. D Gauche conformations are generally higher in energy than anti conformations. HOH Highest Energy CH3 2nd Highest Energy H3C OH 3.
CH 3 CH 3 1 CH. They always have the same functional groups c. They have different chemical properties.
Which of the following conformers has the highest energy is the least stable. Which of the following conformers has the highest energy. Which of the following statements about the conformers that result from rotation about the C2-C3 bond of butane is correct.
A staggered conformation with two larger groups 60 from each other is called anti. 100 5 ratings Confor. Given that the conversion to the lowest energy conformer in Q2 has an equilibrium constant K 20 calculate the percentage of B in solution at 298 K.
In butane the gauche-conformer is less stable than the anti-conformer by about 09 kcalmol. Which conformer is highest in energy. There are two low-energy conformers of cis-decalin R R R and.
This is the highest energy conformation because of unfavorable interactions between the electrons in the front and back C-H bonds. Experts are tested by Chegg as specialists in their subject area. B Staggered conformations are at energy maxima and eclipsed conformations are energy minima.
Eclipsed totally eclipsed gauche and anti. They have different IUPAC names b. IV What is the alternate chair conformation of the following compound.
2 - the one that has the CH3s the closest together. The staggered contormer eclipsed Steric Strain absent In an eclipsed conformer mnore stable than staggered conformet An eclipsed conformer None of the above. Which of the conformers has the highest energy.
A 2 B 3 C 4 D 5 E 6 22 Arrange the following conformers of butane in order of energy lowest to highest. The staggered conformer has a minimum steric energy of 0818 kcal mol because it minimizes the repulsions aforementioned. L would be the furthest apart and this would be the most stable.
C Steric strain is absent in the eclipsed forms. Which of the following conformers has the highest energy. For example trans decalin has one low energy conformer but cis decalin has two.
The increase in the potential energy of the molecule due to increase in the repulsion between the electron clouds of nearby atoms. Rank the conformers of butane in order of decreasing stability putting the most stable first. So our c h three n c.
Gauche conformations are generally higher in energy than anti conformations. Which of the following is the lowest energy conformer of 23-dimethybutane. Order the following moleculesconformers from the most stable to least stable.
We can easily find the stability of the substituted chair confirmation by identifying the steric strain in the given molecules. Among the following conformers which has highest potential energy for mathrmn -butane along. B The gauche conformer is an eclipsed one.
Of the three confirmations of is a vital chloride which is the most stable. IV Which of the following is the lowest energy conformer of 2 3-dimethybutane. Which cycloalkanes has the most angle.
See the answer See the answer See the answer done loading Which of the following conformers has the highest energy the least stable. The first confirmation is the most stable because it has the least steer ex train between the bulky CH three and C L groups as they are the furthest apart in this confirmation. Draw then arrange the following conformers of butane in order of energy lowest to highest.
Soas faras Um numbering these according to the substance Wints. CH3 CH3 - CI CI CH3 CI A B C. If we now rotate the front CH 3 group 60 clockwise the molecule is in the highest energy eclipsed conformation where the hydrogens on the front carbon are as close as possible to the hydrogens on the back carbon.
Entropy is also a consideration when symmetry leads to degeneracy of one conformer eg the enantiomeric conformations of gauche butane. View the full answer. The higher energy of eclipsed bonds is known as eclipsing strain.
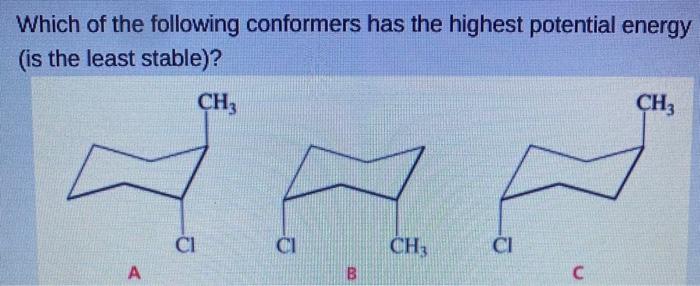
Solved Which Of The Following Conformers Has The Highest Chegg Com
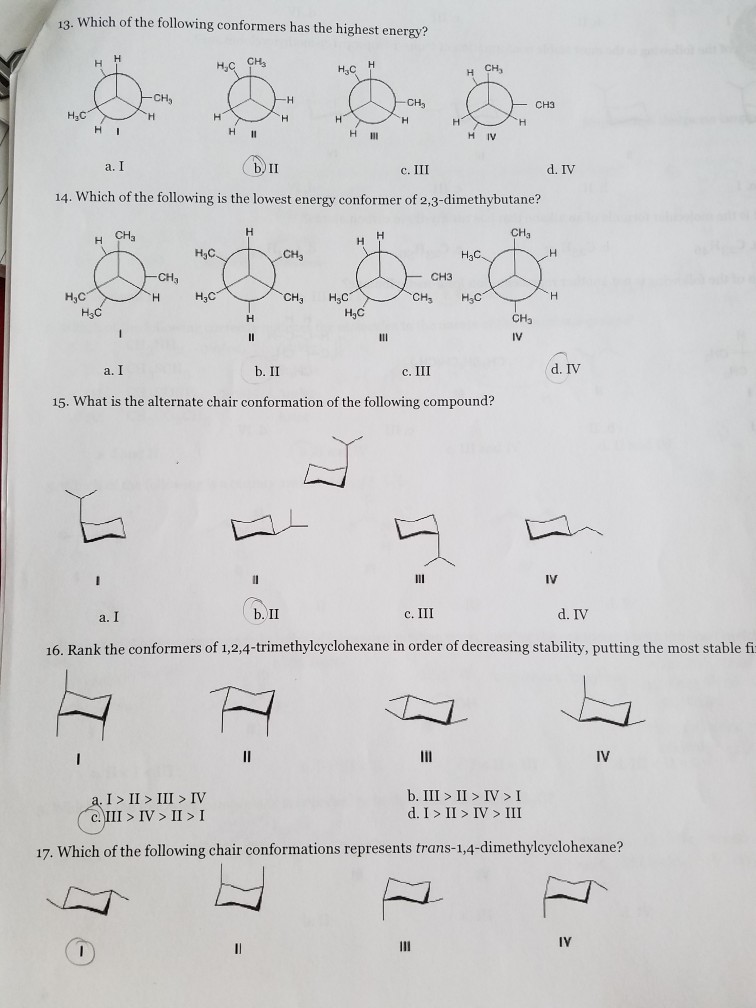
Solved 13 Which Of The Following Conformers Has The Highest Chegg Com
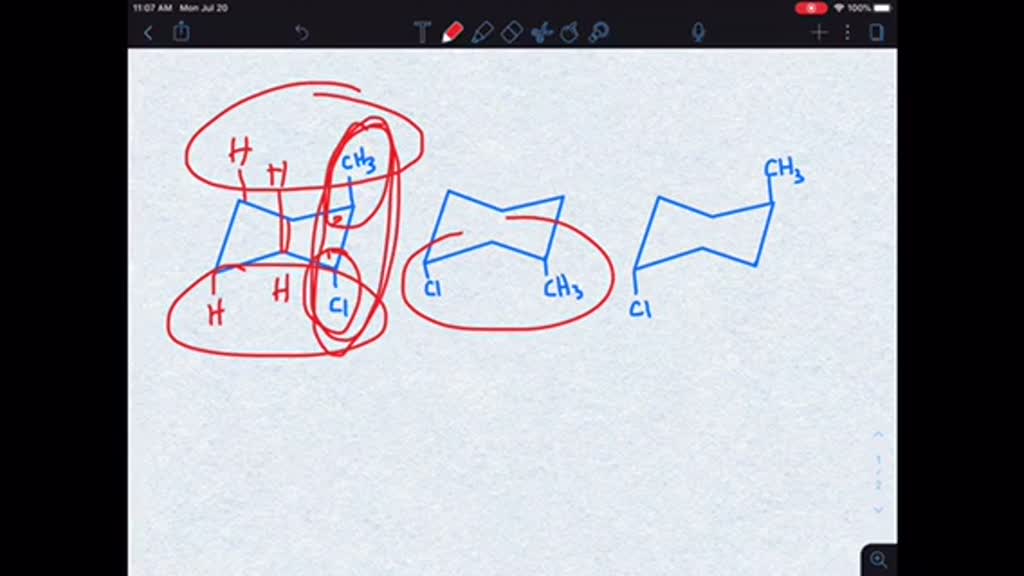
Solved Which Of The Following Conformers Has The Highest Energy Is The Least Stable
Solved Which Of The Following Conformers Has The Highest Studysoup
No comments for "Which of the Following Conformers Has the Highest Energy"
Post a Comment